DTP Branches and Offices
About the Stepping Stones Program
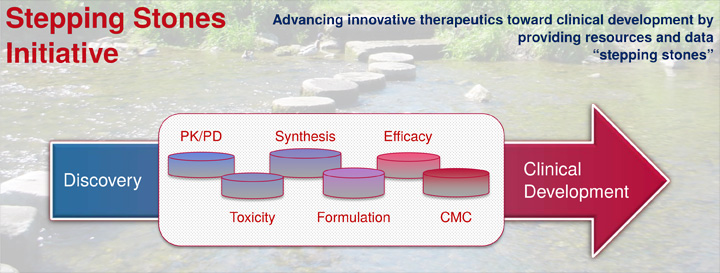
The Stepping Stones Program provides critical resources to advance innovative anti-cancer therapeutics toward clinical development. This initiative is intended to augment grant-supported programs with access to NCI/DCTD/DTP drug development capabilities to fill knowledge and data gaps, thus enabling these programs to advance and procure additional resources for development toward clinical testing. The goals are:
- Support peer-reviewed anti-cancer product development
- Facilitate access to federal resources for preclinical product development
- Fill the NCI/NExT pipeline with innovative therapeutic candidates
Project selection criteria
- Therapeutic candidate development programs supported by NCI/DCTD grant funding
- Well-characterized and validated intervention target/mode of action
- Addresses unmet clinical needs (orphan cancers, glioblastoma, small cell lung cancer, pancreatic cancer, pediatric cancers, etc.)
- Lead candidates with demonstrated preclinical in vitro/in vivo efficacy
Access to Stepping Stones
NCI grantees may submit a Drug Development Consultation Request. A consultation meeting will be arranged for grantees to discuss the most promising grant-supported therapeutic candidate with a panel of NCI staff with therapeutic development expertise. Grantees with qualified research projects may be invited for further discussion with NCI/DCTD/DTP staff to identify research gaps and opportunities for NCI to perform a discrete set of studies to address the most critical product development gap. The scope of studies is preclinical; IND-enabling support (GLP, GMP) is not provided.





