DTP Branches and Offices
Success Story
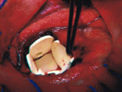
Gliadel Wafers being implanted in the brain. Courtesy of NCI. 1997.
Carmustine (BCNU, NSC 409962)
The NCDDG helped develop a new form of Carmustine, a successful anticancer agent. The new product was a BCNU-impregnated wafer that is implanted at the time of surgical resection of the tumor.
1962
Carmustine (BCNU, NSC 409962) was synthesized by Southern Research Institute through a contract with CCNSC. It was accepted for clinical development in 1962 because of its superior activity compared with two analogs in the mouse leukemia tumor model L1210.1
BCNU acts as an alkylating agent although biochemical and microbiological differences exist between the drug and other alkylating agents. In animal models, BCNU is most effective when administered by intraperitoneal injection daily and is active by several schedules when given orally. Animal toxicology studies have shown that the most dramatic and consistent toxicities involve the bone marrow, lymphoid tissue, liver, kidney, and gastrointestinal tract. In clinical trials, BCNU has been shown to cross the blood-brain barrier and be effective against previously untreated central nervous system leukemia. Its dose-limiting factor is delayed hematopoietic toxicity.
1996
In 1996, a new form of BCNU was approved for use in addition to surgery to prolong survival in patients with recurrent glioblastoma multiforme who qualify for surgery. This agent, called Gliadel Wafer, was developed by NCDDG, headed by Henry Brem of Johns Hopkins University. The product consists of BCNU impregnated in a wafer composed of a polyanhydride biodegradable polymer invented by Robert Langer of the Massachusetts Institute of Technology.2 Wafers are implanted at the time of surgical resection of the tumor. Over time, the drug diffuses away from the polymer, which eventually degrades. The product is marketed in the United States as well as in Brazil, Canada, and several European countries.
1 DeVita VT, Denham C, Davidson JD, Oliverio VT. The physiological disposition of the carcinostatic 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) in man and animals. Clin Pharmacol Ther 1967;8:566–577.
2 Sipos EP, Tyler B, Piantadosi S, Burger PC, Brem H. Optimizing interstitial delivery of BCNU from controlled release polymers for the treatment of brain tumors. Cancer Chemother Pharmacol 1997;39:383–389.





