DTP Branches and Offices
Success Story
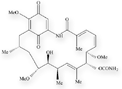
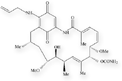
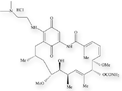
Molecular structures of geldanamycin and its analogs, 17-AAG and 17-DMAG.
Geldanamycin (NSC 122750)
17-AAG (NSC 330507)
17-DMAG (NSC 707545)
Although 17-AAG and 17-DMAG both exhibit activity in numerous tumor models, only 17-DMAG is active when given orally.
1968
Heat shock protein 90 (Hsp90) is an abundant molecular chaperone that plays a major role in the folding and activation of several signaling proteins that promote the growth and survival of tumor cells. Geldanamycin, originally submitted by the Upjohn Company, induces the degradation of these signaling proteins and, consequently, the death of the malignant cells. Although geldanamycin was active in preclinical studies, it was a poor candidate for clinical trials due to its in vivo toxicity and instability.
1980
DTP has worked on several geldanamycin derivatives in an effort to develop nontoxic, more readily soluble analogs of the drug.
1992
17-allylamino demethoxygeldanamycin (17-AAG) is an analog of geldanamycin. This drug has been undergoing phase I/II clinical trials since 1999. 17-AAG is an ansamycin derivative that binds specifically to Hsp90 in a manner similar to that of geldanamycin itself. 17-AAG also leads to degradation of several Hsp90-induced signaling proteins. Even though Hsp90 binding by 17-AAG is weaker than binding by geldanamycin, 17-AAG and geldanamycin cause biologic effects in tumor cells at similar doses. Since 17-AAG has a better toxicity profile than geldanamycin, it is clearly a more engaging clinical candidate. Preliminary results indicate that the target dose of this analog can be achieved without dose-limiting toxicity.
1997-1998
17-dimethylaminoethylamino demethoxygeldanamycin hydrochloride (17-DMAG), another geldanamycin analog, has excellent bioavailability, is widely distributed to tissues, and is quantitatively metabolized much less than is 17-AAG.1
2002-2004
17-DMAG is being studied for treating patients with solid tumors or lymphomas.2
1 Invivogen.
2 ClinicalTrials.gov. Identifier No. NCT00086008.
Link:
17-AAG poster (pdf)





