DTP Branches and Offices
**Important Updates**
Currently, the NCI is not accepting submissions of compounds for NCI-60 testing submitted from investigators outside of the United States and U.S. Territories.
The NCI has modernized the methodology for the NCI-60 Human Tumor Cell Lines Screen, which includes a change to 384-well plates and a luminescent readout.
- September 30, 2023 was the last day to submit compounds for testing using the traditional platform.
- Compounds submitted after September 30, 2023 will be tested in the modernized NCI-60 HTS384 format.
Announcement: Update to NCI-60 (August 2023)
Why Has the NCI Modernized the NCI-60?
The NCI-60 Human Tumor Cell Lines Screen has been an integral research resource for anti-cancer drug discovery and development investigators for more than 30 years, with more than 110,000 total compounds screened in 260,000 assays.
NCI has developed a new NCI-60 platform comprising an automated high-throughput assay system conducted in 384-well plates. This modernization is consistent with an assay readout that most investigators use and will improve operational efficiency.
Samples received after September 30, 2023, will be tested in the modernized NCI-60 HTS384 platform.
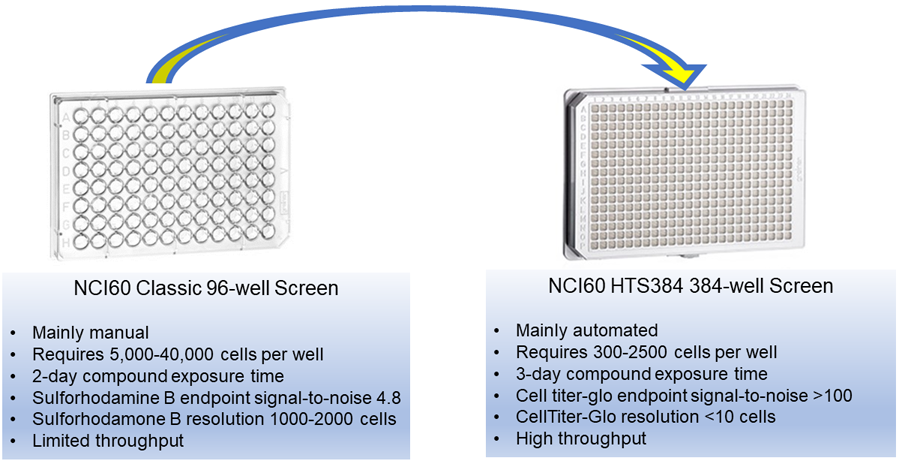
Comparison of NCI-60 Classic 96-well and the Modernized NCI-60 HTS384 Screening formats
What Remains Unchanged?
- Cell lines
- Growth media
- Workflow (Initial screening will be performed at a single concentration followed by a potential screen across five concentrations depending on activity)
What are the New/Optimized Parameters?
- Number of cells plated
- Test compound exposure time (increased from 2 to 3 days)
- Detection endpoint (changed to CellTiter-Glo® luminescence)
Are there Baseline Data for the New Platform?
Yes. To generate the same set of baseline data for the new HTS384 platform that are available for the traditional 96-well platform, we assayed 1,000 compounds from the Approved and Investigational agents’ library.
For most compounds, mean-graph patterns and COMPARE analyses are very similar between the two assays. A manuscript describing the results is in preparation.
What is the Timeline for the Change to the New Screen?
- NCI accepted new compounds through September 30, 2023 for the classic NCI-60 format.
- All compounds submitted after September 30, 2023 will be entered into the queue for testing in the new NCI-60 HTS384 format.
NCI-60 Human Tumor Cell Lines Screen
- Main
- Announcement: Update to NCI-60 (August 2023)
- Compound Submission
- Data Retrieval and Testing Decisions
- List of NCI-60 Human Tumor Cell Lines
- Screening methodology (One-dose and Five-dose Assay)
- Sample Handling and Preparation
- Database of Screening Results
- Molecular Characterization of NCI-60
- Publications





